Low Energy Ion Scattering Mass Spectrometer (LEIS)
Instrument specification
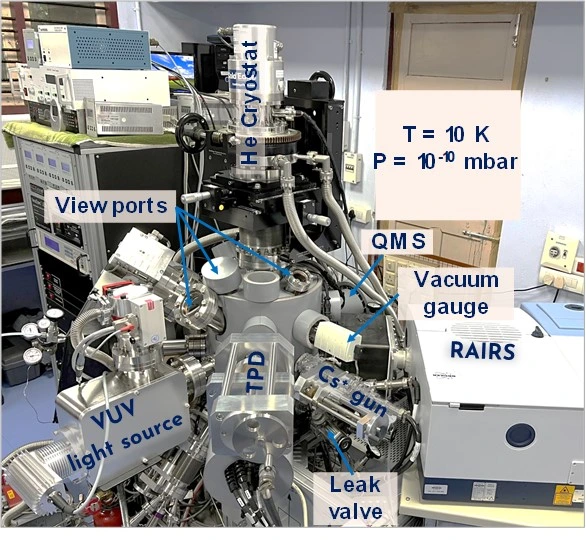
Technical Capabilities
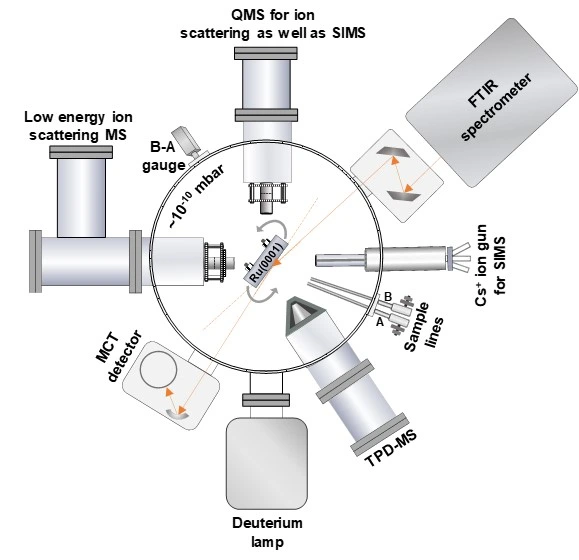
This instrument is designed to study ice surfaces relevant to terrestrial and extra-terrestrial environments. The basic conditions prevailing in outer space are ultrahigh vacuum with a base pressure range <5 x 10-10 mbar, and low temperatures, in the range of 5-200 K. The whole instrument is maintained at ultrahigh vacuum with several vacuum pumps connected in series. There is a target substrate of Ru(0001) single crystal (Princeton Scientific Corp.) inside the chamber which can be cooled to a temperature as low as 8 K using a closed cycle helium cryostat connected with a temperature controller and heater. It can maintain any temperature of interest. Thin layers of various molecular solid (ice) surfaces, such as water, alcohols, carbon monoxide etc., can be created by vapor depositing on the precooled single crystal substrate. The reactivity of such a molecular solid surface is probed by various techniques.
Low energy ion scattering mass spectrometry is one of those. This technique uses reactive ions produced by electron impact ionization (EI) which are then mass and energy selected by quadrupole mass analyzer and subjected to collide on the desired molecular solid surfaces. The scattered ions from the surfaces are analysed by another quadrupole mass spectrometer. Together with this, we have a temperature-programmed desorption mass spectrometer (TPD-MS) to probe the surface desorbing species, a low-energy alkali-ion gun to trigger out the neutral species present on the surface, and a reflection absorption infra-red spectrometer (RAIRS) for getting the infra-red spectrum of surface species. To study the photochemistry of the ice, this instrument is equipped with a vacuum ultraviolet source (Deuterium lamp with magnesium fluoride window).
Schematic of the ice instrument
Key features of LEIS technology
- Low energy ion scattering mass spectrometer (LEIS-MS)
- Temperature-programmed desorption mass spectrometer (TPD-MS)
- Reflection Absorption Infrared Spectrometer (RAIRS)
- Low energy alkali ion gun (Cs+/Li+)
- Metal evaporator
- Vacuum Ultraviolet (VUV) light (Deuterium lamp)
Ultra Low Energy Ion Scattering Mass Analyser
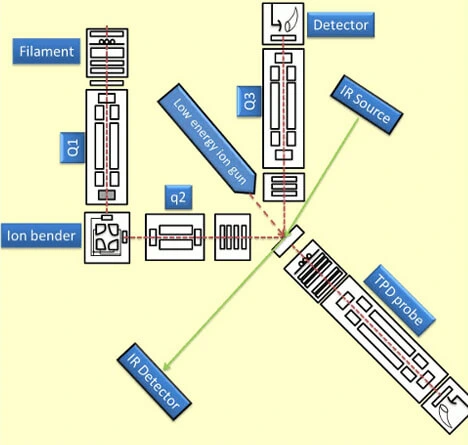
This instrument is designed to study ice surfaces relevant to terrestrial and extraterrestrial environment. The basic conditions prevailing there in outer space are ultra high vacuum (base pressure range < 5 x 10-10 mbar) and low temperature of the range of 5-200 K. The whole instrument maintained at ultra high vacuum (UHV) with several vacuum pumps connected in series. There is a target of Ru(0001) single crystal (Princeton Scientific Corp.) inside the chamber which can be cooled to a temperature as low as 8 K using a closed cycle helium cryostat connected with a temperature controller and heater to maintain any temperature of interest. Thin layers of various molecular solid (ice) surfaces such as water, alcohols, carbon monoxide etc. are developed by vapour depositing on the precooled single crystal substrate. The reactivity of such a molecular solid surfaces probed by various techniques. Low energy ion scattering mass spectrometry is one of those. The technique uses reactive ions produced by electron impact ionization (EI) which are then mass and energy selected by quadrupole mass analyzer and subjected to collide on the desired molecular solid surfaces. The scattered ions from the surfaces are analysed by another quadrupole mass spectrometer. Together with this we have a temperature programmed desorption mass spectrometer (TPD-MS) to probe the surface desorbing species, low energy alkali-ion gun to trigger out the neutral species present on the surface, Reflection absorption infra-red spectrometer (RAIRS) for getting the infra-red spectrum of surface species.
Source of the components
- Vacuum components: Pfeiffer Vacuum GmbH, Germany
- Reflection absorption IR spectrometer: Bruker Optik GmbH, Vertex 70
- Low energy alkali-ion gun: Kimball Physics Inc, USA
- Closed cycle He cryostat: ColdEdge Technologies, USA
- Deuterium source: McPherson, USA
Notable mentions from our group
References
- Clathrate hydrates in interstellar environment, Jyotirmoy Ghosh, Rabin Rajan J. Methikkalam, Radha Gobinda Bhuin, Gopi Ragupathy, Nilesh Choudhary, Rajnish Kumar, and Thalappil Pradeep, Proc. Natl. Acad. Sci. U.S.A., 116 (2019) 1526-1531 (DOI: 1073/pnas.1814293116).
- Formation and Transformation of Clathrate Hydrates under Interstellar Conditions, Jyotirmoy Ghosh, Gaurav Vishwakarma, Rajnish Kumar, and Thalappil Pradeep, Acc. Chem. Res., 56 (2023) 2241–2252. (DOI: 10.1021/acs.accounts.3c00317)
- Formation of cubic ice via clathrate hydrate, prepared in ultrahigh vacuum under cryogenic conditions, Jyotirmoy Ghosh, Radha Gobinda Bhuin, Gaurav Vishwakarma, and Thalappil Pradeep, J. Phys. Chem. Lett., 11 (2020) 26-32 (DOI: 1021/acs.jpclett.9b03063).
- Iron assisted formation of CO2 over condensed CO and its relevance to interstellar chemistry, Rabin Rajan J. Methikkalam, Jyotirmoy Ghosh, Radha Gobinda Bhuin, Soumabha Bag, Gopi Ragupathy, and Thalappil Pradeep, Phys. Chem. Chem. Phys., 22 (2020) 8491-8498 (DOI: 1039/c9cp06983f).
- Interaction of acetonitrile with water-ice: An infrared spectroscopic study, Radha Gobinda Bhuin, Rabin Rajan J. Methikkalam, Bhalamurugan Sivaraman and Thalappil Pradeep, J. Phys. Chem. C, 119 (2015) 11524-11532 (DOI: 1021/jp512607v).
- Development of ultralow energy (1-10 eV) ion scattering spectrometry coupled with reflection absorption infrared spectroscopy and temperature programmed desorption for the investigation of molecular solids, Soumabha Bag, Radha Gobinda Bhuin, Rabin Rajan J. Methikkalam, Luke Kephart, Jeff Walker, Kevin Kuchta, Dave Martin, Jian Wei and Thalappil Pradeep, Rev. Sci. Instrum., 85 (2014) 014103 (DOI: 1063/1.4848895).
- Induced migration of CO2 from hydrate cages to amorphous solid water under ultrahigh vacuum and cryogenic conditions, Gaurav Vishwakarma, Bijesh Malla, Karri Sesha Surya Vara Prasad Reddy, Jyotirmoy Ghosh, Soham Chowdhury, Sharma S. R. K. C. Yamijala, Sandeep Kumar Reddy, Rajnish Kumar, and Thalappil Pradeep, Phys. Chem. Lett. 2023, 14 2823–2829 (DOI: 10.1021/acs.jpclett.3c00373).
- Simulated interstellar photolysis of N2O ice: Selectivity in photoproducts, Bijesh Malla, Soham Chowdhury, Devansh Paliwal, Hanoona K. M., Gaurav Vishwakarma, Rabin Methikkalam and Thalappil Pradeep, J. Phys. Chem. C. (2024) (DOI: 10.1021/acs.jpcc.4c06624)
- Dissociation and reformation of CO2 clathrate hydrate cages in amorphous ice thin film under ultrahigh vacuum, Bijesh Malla, Soham Chowdhury, Gaurav Vishwakarma, Rajnish Kumar and Thalappil Pradeep, J. Phys. Chem. Lett. (2025) (DOI: 10.1021/acs.jpclett.5c00393)
- Composition-dependent formation and dissociation of structure I and structure II clathrate hydrates of trimethylene oxide in ultrahigh vacuum, Soham Chowdhury, Bijesh Malla, Gaurav Vishwakarma, Anupriya Nyayban and Thalappil Pradeep, J. Phys. Chem. C. (2025) (DOI: 10.1021/acs.jpcc.5c01603)

