Rotary Semi-Automatic Microtome
A microtome Is a tool used to cut extremely thin slices of material, known as ‘sections’. These microtome sections are used microscopy for the preparation of samples for observation under transmitted light or electron radiation. Microtome use steel, glass or diamond blades depending upon the specimen being sliced and the desired thickness of the sections being cut. In light microscopy, steel blades are commonly used to prepare sections of animal and plant tissues. Glass knives are used to slice sections for light microscopy. Industrial grade diamond knives are used to slice hard materials such as bone, teeth and plant matter for both light microscopy and electron microscopy. This equipment is made by Medimeas, India.

History of microtome:
In the beginnings of light microscopy development, sections of plant and animal tissues were manually cut using razor blades. Later, it was observed that to analyze the structure of tissue specimens, it was important to make clean, reproducible cuts on the order of 100um, through which light can be transmitted. This allowed for the observation of samples using light microscopes in a transmission mode. The first device for the preparation of such cuts was invented by George Adams Jr in 1770 and further developed by Alexander Cummings. The table-top based microtome model was developed by Andrew Prichard in 1835. Later, some more improved version of microtome was developed by anatomist Wilhelm His Sr in 1865.
Medimeas semi-automatic rotary microtome:
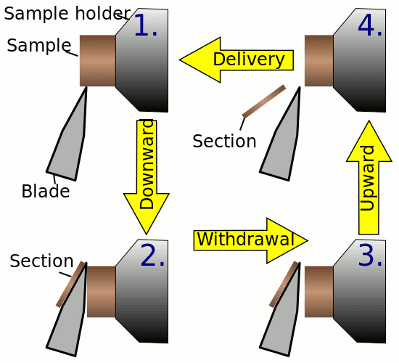
In a rotary microtome, the knife is typically fixed in a horizontal position. This device operates with a staged rotary action such that the actual cutting is part of the rotary motion. The rotary microtomes are the most common form of microtome design. The other types of micro tomes commercially available are vibrating microtome, saw microtome, sledge microtome and laser microtome. In the figure the principle of the cut is explained. The typical cut thickness of a rotary microtome is between 1 and 60 um. In hard materials, such as a sample embedded in a synthetic resin, this design of microtome can allow for a good ‘semi-thin’ sections with a thickness of as low as 0.5um.
Fixing, dehydration and processing of animal tissues:
The usual fixative for paraffin embedded tissues is neutral buffered formalin (NBF). Optimal histology requires adequate fixation of animal tissues for about 48 hours at room temperature for thinly sliced tissues of 30mmx20mmx3mm (thickness). Other commonly used fixatives include formaldehyde, ethanol, methanol and/or picric acid. The processing of animal tissue is done after fixing of tissues. In tissue processing procedure, it is dehydrated through a series of graded alcohol (70%, 95%, and 100%) over a period of 8-10 hours and then cleared with a clearing agent like Xylene over a period of 2 hours.
Paraffin infiltration and sectioning of biological tissue samples:
Biological materials are usually placed in a more rigid fixative in a process known as ’embedding’ of the sample before cutting by microtome. This is achieved by the inflow of a liquid substance around the sample such as paraffin(wax) or epoxy resin, which is placed in a mould and later hardened to produce a ‘block’ which is readily fixed on the sample holder and cut. Paraffin wax can be purchased with a melting points at different temperatures, the most common for histological use being about 56-58 C. Once the tissue is embedded, it is stable for many years. The embedding process of tissues is done to increase the mechanical strength and stability and make them easier to cut into thin slices.
Staining of plant and animal tissues:
Staining is an auxiliary technique used in microscopy to enhance contrast in the microscopic image. Staining is not limited to biological materials, it can also be used to study the morphology of other materials like structure of semi-crystalline polymers or the domain structures of block copolymers. Other living microorganisms like bacteria, fungi and virus are in-vivo stained before examining them on a microscope.


