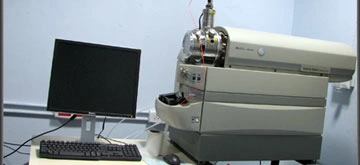
Travelling Wave Ion Mobility Mass Spectrometer: SYNAPT G2-Si High Definition MS (HDMS) System
Instrument Specification:
Waters Synapt G2-Si high definition mass spectrometry uses high efficiency traveling wave (T-wave) ion mobility with high performance tandem MS to significantly enhance the peak capacity, specificity, and sensitivity of the analysis. When mass resolution and chromatography are not enough, high-efficiency T-wave ion mobility gives an additional dimension of separation, based on molecular size and shape.

Technicalities:
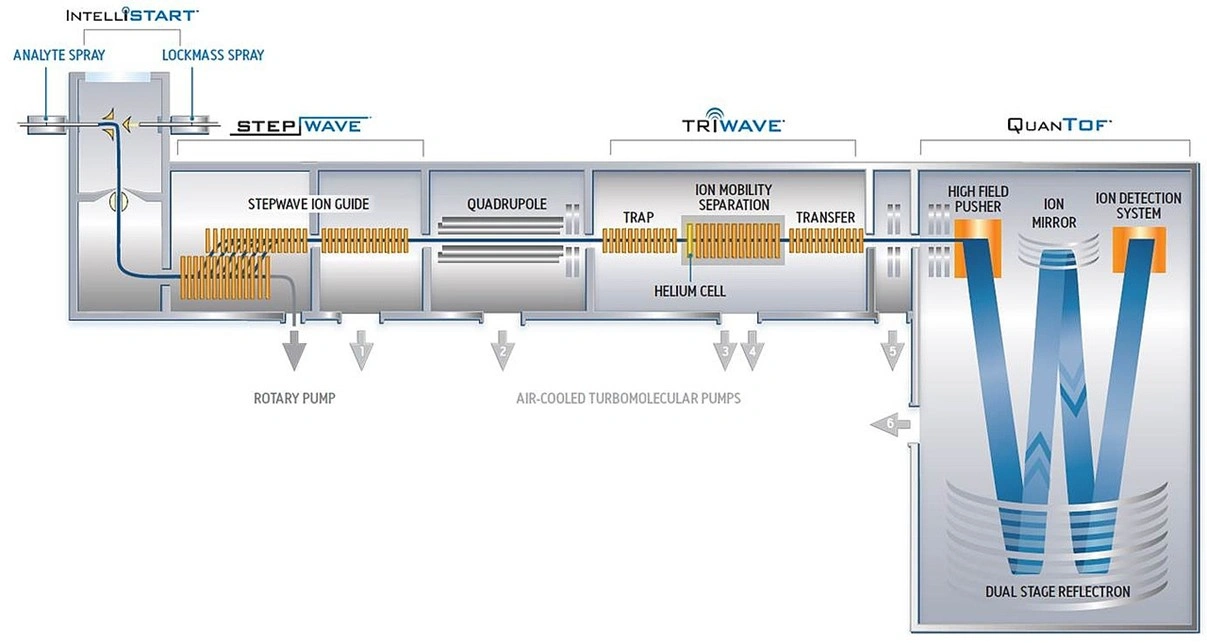
The architecture of Synapt’s ion source interfaces with a huge range of techniques, from separation with UPLC, to ionization with ESI, APCI, ESCI. For direct analysis methods, T-Wave IMS provides the highest separation and selectivity, post-ionization. This is particularly advantageous when combined with the high performance MALDI option, which can be rapidly switched with atmospheric pressure ion sources. The resolving quadrupole is available in 8KDa or 32KDa mass range options for MS/MS of small to macromolecular species.
The dual collision cell arrangement of TriWave enhances MS/MS possibilities, by providing Collision Induced Dissociation (CID) and/or Electron Transfer Dissociation (ETD) fragmentation with high resolution and accurate mass measurements. It is coupled with time of flight (TOF) technique. It operates in TOF mode and HDMS mode. The TOF mass range is 50 to 100,000 m/z in Resolution mode, and 50 to 32,000 m/z in High Resolution mode.
Key Features of QuanTof Technology:
- Over 50,000 FWHM mass resolving power
- Exact mass (<1 ppm RMS)
- Accurate Isotope Abundances
- In-spectrum dynamic range and linearity in detector response of up to 106
- Up to 30 spectra data acquisition per sec
Key Features of QuanTof Technology:
- Over 50,000 FWHM mass resolving power
- Exact mass (<1 ppm RMS)
- Accurate Isotope Abundances
- In-spectrum dynamic range and linearity in detector response of up to 106
- Up to 30 spectra data acquisition per sec
Schematic of the Waters Synapt G2-Si instrument*
- For more information, visit water@abcd.com
Theory of operation:
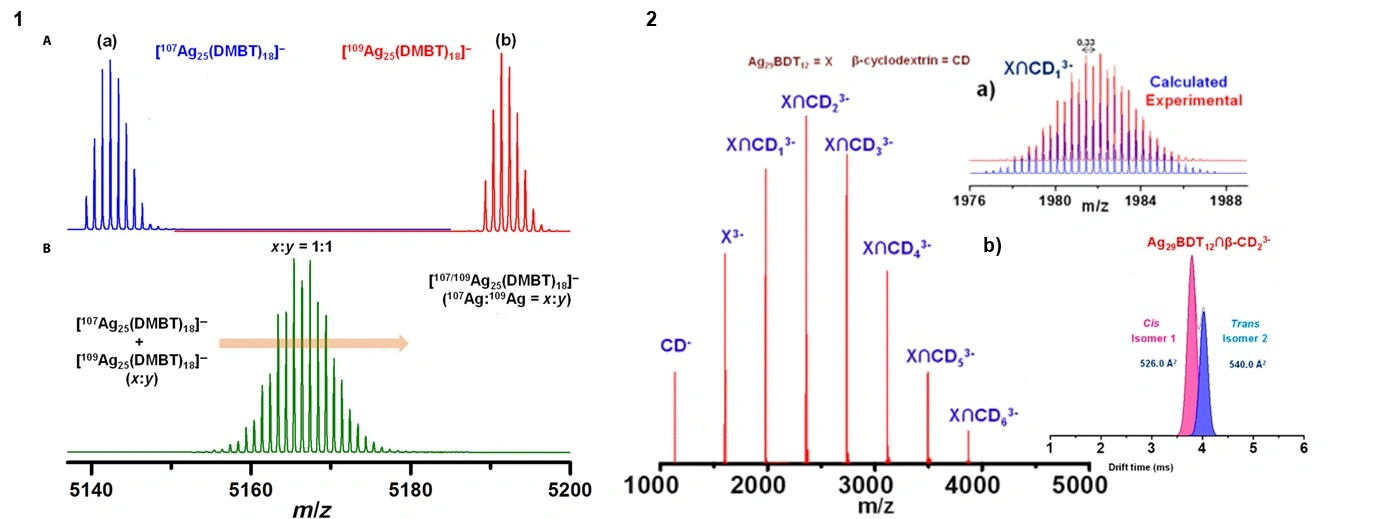
Figure. (A) ESI MS of the isotopically pure clusters, (a) [107Ag25(DMBT)18]− and (b) [109Ag25(DMBT)18]−. (B) Mass spectral distribution of the product obtained by mixing the two isotopic clusters at 1:1 molar ratio. 2. ESI MS of [X∩(CD)n]3− (n = 1 to 6) supramolecular complexes, where X and CD represent Ag29(BDT)12 and β-cyclodextrin. (a) Theoretical and experimental isotopic patterns for [X∩(CD)n]3−. (Adapted and modified from ref 3 as well as from ref 4)
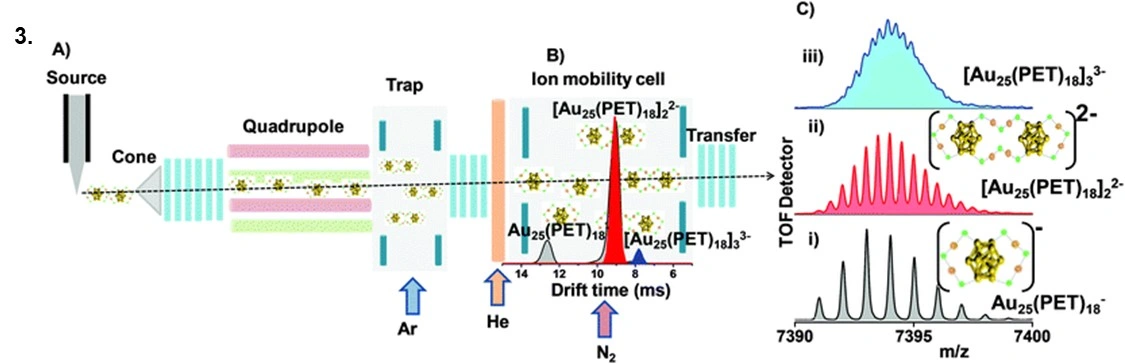
Notable mentions:
Further details are available in the following references:
- SYNAPT G2-Si Mass Spectrometry | Waters (accessed May 14, 2021).
- Enabling Greater Capability in the Characterization of Biomolecules with the Incorporation of Novel Off-Axis StepWave Ion Transfer Optics in the High Resolution SYNAPT G2-S System : Waters (accessed May 14, 2021).
- Chakraborty, P.; Nag, A.; Natarajan, G.; Bandyopadhyay, N.; Paramasivam, G.; Kumar Panwar, M.; Chakrabarti, J.; Pradeep, T. Rapid Isotopic Exchange in Nanoparticles; 2019.
- Nag, A.; Chakraborty, P.; Paramasivam, G.; Bodiuzzaman, M.; Natarajan, G.; Pradeep, T. Isomerism in Supramolecular Adducts of Atomically Precise Nanoparticles. J. Am. Chem. Soc 2018, 140, 13590–13593. https://doi.org/10.1021/jacs.8b08767.
- Baksi, A.; Chakraborty, P.; Bhat, S.; Natarajan, G.; Pradeep, T. A Noble Metal Cluster Dimer in the Gas Phase † ChemComm COMMUNICATION. Chem. Commun 2016, 52, 2. https://doi.org/10.1039/c6cc03202h.